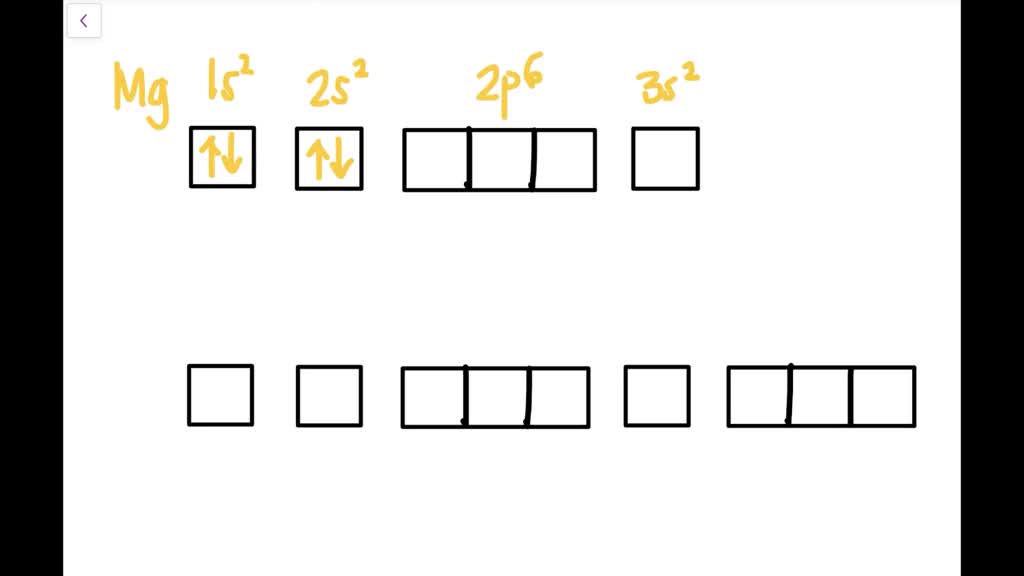
orbital diagram for magnesium
Interactive Periodic Table Let me tell you how this Interactive. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital.
Solved Atomic Test 1 Write The Electron Configuration And Orbital Diagrams For The Following Species A Mg No Short Cut B Ru C Si 1 2a Write The Lewis Structures For The Following
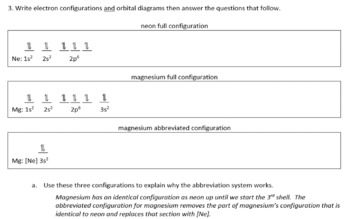
The orbital notation of magnesium is 1S2 2S2 2P6 3S2.

. Magnesium orbital diagram According to Hunds principle the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise. Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. Magnesium has an atomic number 12 and an atomic mass number 24 how many electrons are in its third energy shell.
The nex six electrons will go in the 2p orbital. Orbital diagram Magnesium electron configuration Electronic configurations of elements Mg Magnesium is an element with position number 12 in the periodic table. An atomic number of 12.
1 Magnesium is a metal that possesses an atomic number of 12. The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom it uses an arrow to represent the electrons every orbital one box contains a maximum. Free Gift for you.
The orbitals are 1s 2s 2p and 3s. The orbital diagram uses up or down arrows to symbolize the electron. What is the orbital diagram for Magnesium Mg.
The orbital diagram of magnesium shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons the 2p subshell has 6 electrons and the 3s subshell has 2. The orbital diagram for Magnesium is drawn with 4 orbitals. An atomic orbital is a type of orbital.
How to Write the Orbital Diagram for Magnesium Mg 3385 views Dec 6 2021 To write the orbital diagram for the Magnesium atom Mg first we need to write the electron. The Magnesium orbital diagram contains 2. The p orbital can hold up to six electrons.
Hence the electrons found in the M-shell of the Magnesium. Depict the electron configuration for magnesium ing an orbital box diagram and noble gas notation. Bohrs diagram of Magnesium has three electron shells K L and M the inner shell is the K-shell and the outermost shell is M-shell.
Located in the III.
What Fraction Of The Orbitals In 1 Mol Of Mg Atoms In A Metallic Network Are Occupied At 0 K Socratic
Electron Configuration With Examples Online Chemistry Tutorials
Electron Configuration And Orbital Diagram Practice Tpt

Give The Orbital Diagram Of The Following A Magnesium Chloride B Nitrogen C Methane D Hydrogen Chlor
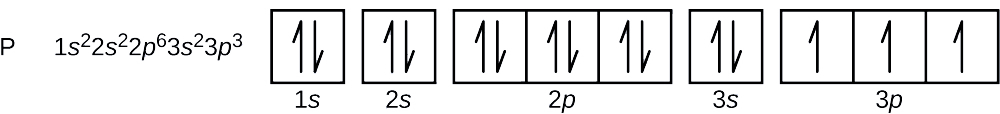
8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts
Magnesium Oxide

Write The Electron Configuration For Magnesium A 12 And Potassium A 19 Draw Their Orbital Brainly Ph
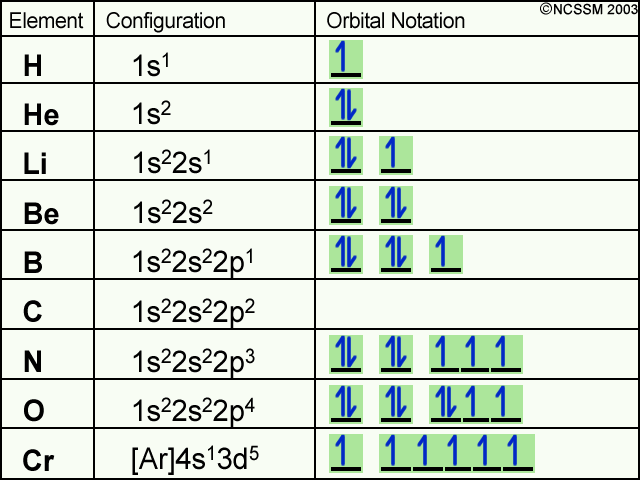
Does The Electron Configuration Of An Atom Tells Us How Many Electrons Are In Each Orbital Socratic

4 6 Electronic Configuration The Atom Siyavula
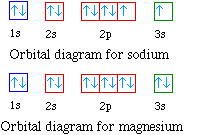
Arrangements Of Electrons In The Orbitals Of An Atom Is Called Its Electron Configuration
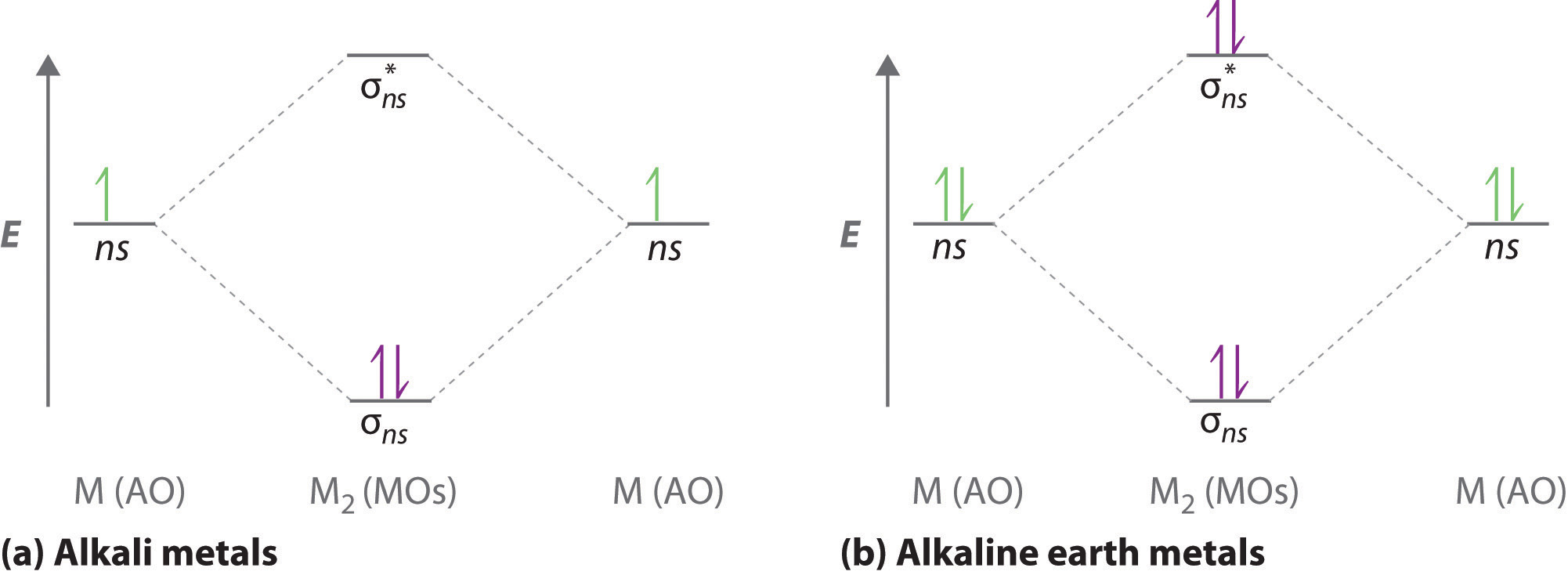
Delocalized Bonding And Molecular Orbitals

Magnesium Orbital Diagram Electron Configuration And Valence Electrons
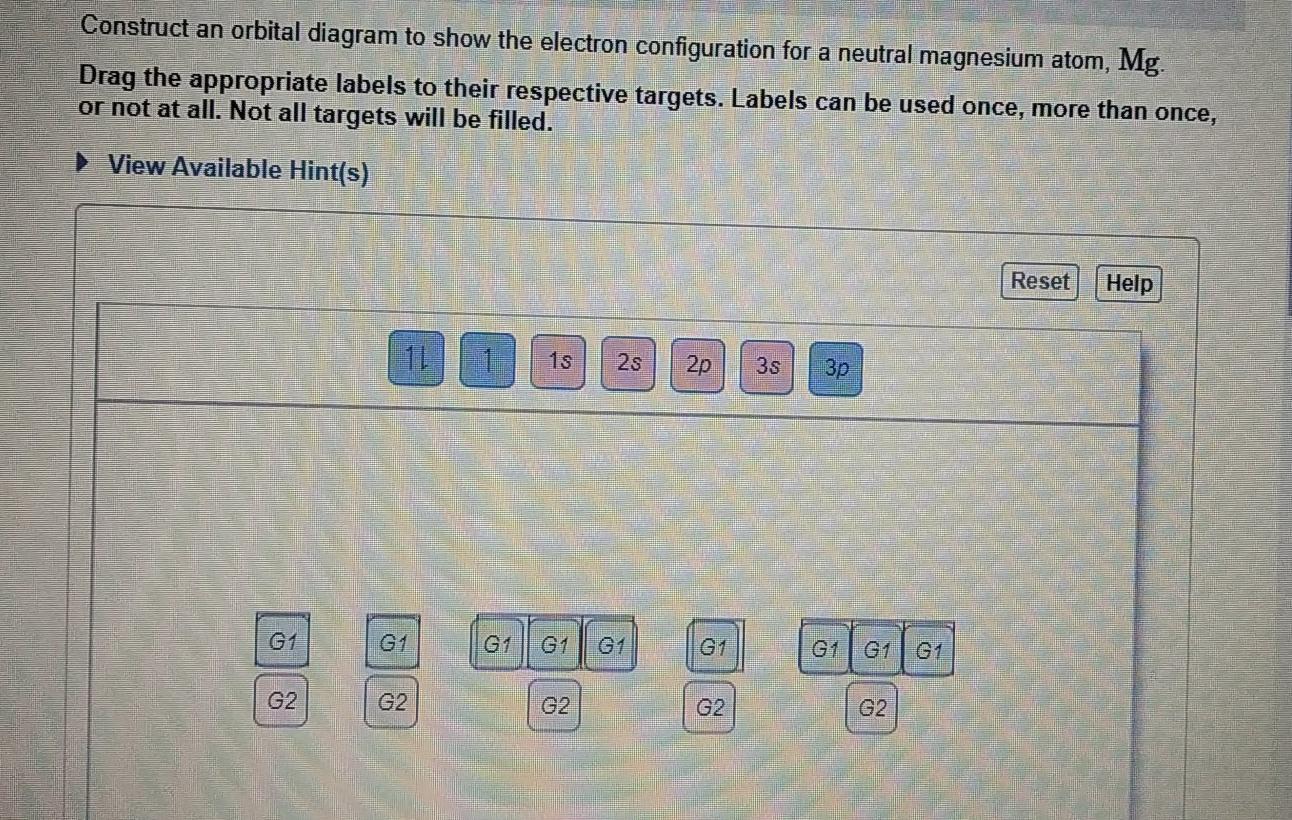
Solved Construct An Orbital Diagram To Show The Electron Chegg Com
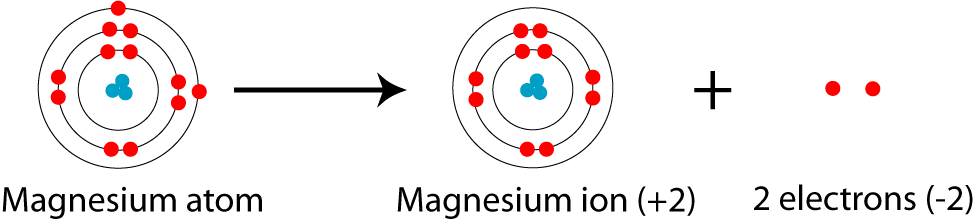
Electronic Configuration Of Magnesium Cation Mg Bob Cut Magazine

Practical Application For Drawing Electron Orbital Diagrams Study Com
What Is The Orbital Diagram For Chromium Quora
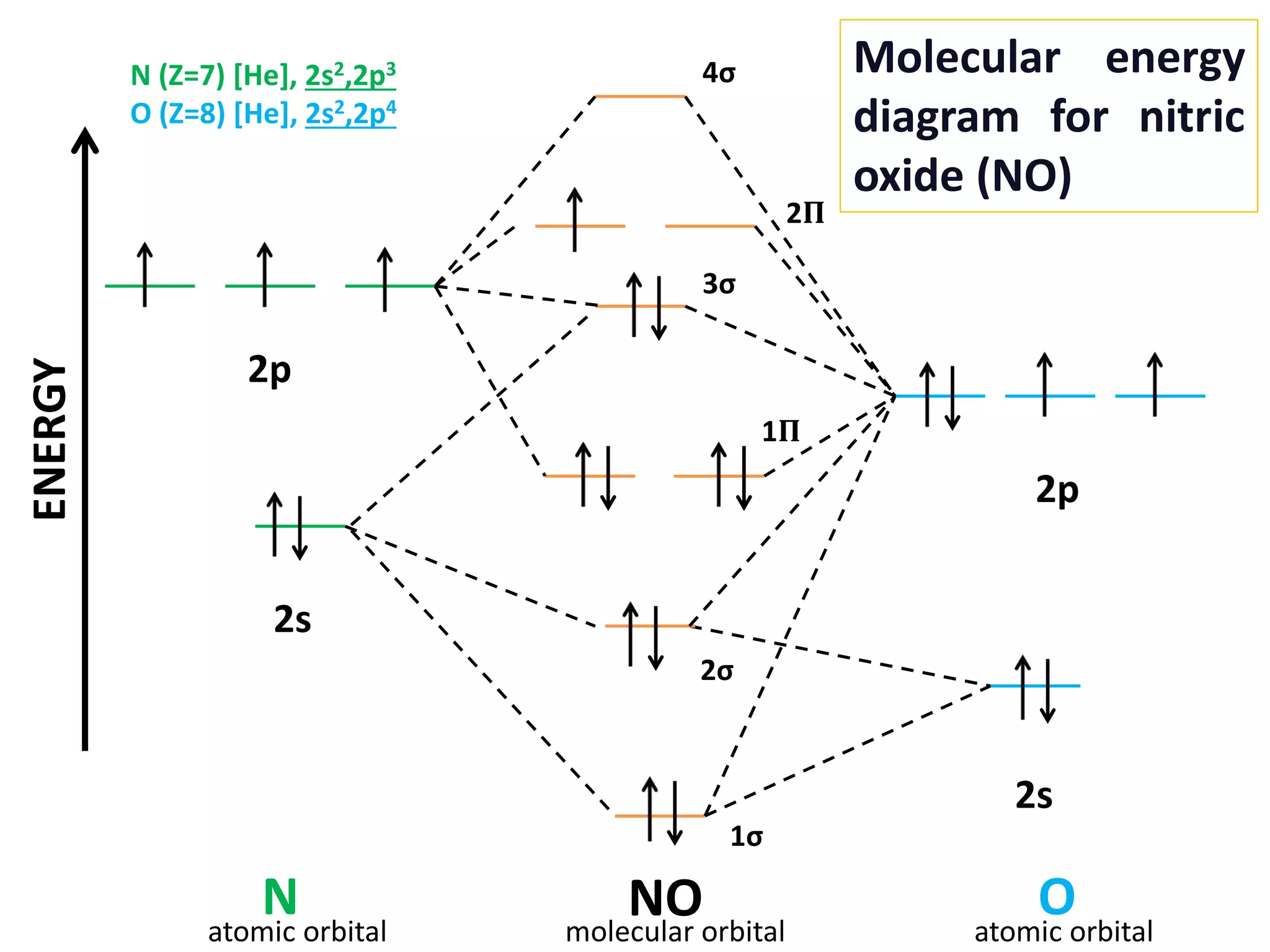
Molecular Orbital Diagram Of Co And No